VSEPR Chart: Your Simple Guide To Molecular Shapes
Ever looked at a chemical formula and wondered what the molecule actually looks like in three dimensions? It's a common thought, isn't it? Knowing the shape of a molecule isn't just for show; it really helps us figure out how chemicals will act, how they might react with other things, or even why some substances are gases while others are liquids. This is where the amazing VSEPR chart comes into play, a truly helpful tool for anyone trying to get a clearer picture of molecular structures.
For students, especially those just starting out with chemistry, figuring out molecular shapes can seem a bit tricky at first. You might be drawing Lewis structures, like for CH4 or H2O, and then thinking, "Okay, but what does it really look like?" That's a very good question to ask, and the VSEPR chart is designed to give you that visual answer. It helps you see beyond the flat drawing and imagine the actual 3D form, which is, you know, pretty cool.
So, whether you're trying to draw a 3D diagram, classify a shape at a central atom, or even predict how many molecules are nonpolar, having a solid grasp of the VSEPR chart makes a big difference. It's a way to organize what we know about electron arrangements around a central atom, and it makes predicting shapes much more straightforward. You'll soon see how it works, and it's quite a helpful way to approach these chemical puzzles, that's for sure.
Table of Contents
- What is VSEPR Theory?
- How the VSEPR Chart Helps
- Common Molecular Shapes from the VSEPR Chart
- Putting the VSEPR Chart to Work: Examples
- Beyond Shape: Bond Angles and Polarity
- Practical Tips for Using Your VSEPR Chart
- Frequently Asked Questions About the VSEPR Chart
- A Final Thought on the VSEPR Chart
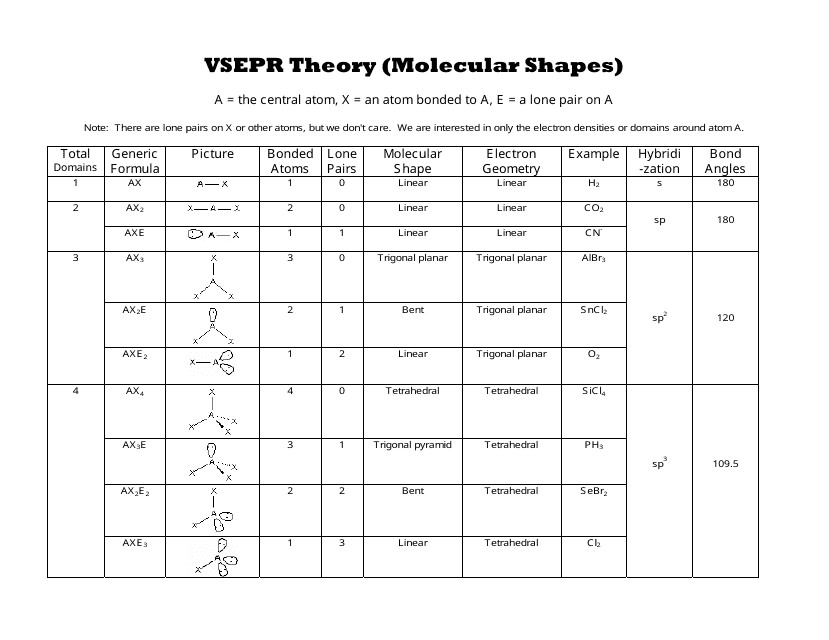
What is VSEPR Theory?
The Basic Idea
VSEPR stands for Valence Shell Electron Pair Repulsion. It's a pretty smart idea that helps us guess the three-dimensional arrangement of atoms in a molecule. The main thought behind it is quite simple: electron groups around a central atom will try to get as far away from each other as they can. This happens because electrons, which have a negative charge, push away from other electrons. So, in a way, they're always trying to find their personal space.
This pushing away, or repulsion, is what makes molecules take on specific shapes. Think about it: if you have a few balloons tied together at a central point, they naturally spread out to give each other room. Molecules do something very similar with their electron groups, and that's how we get shapes like linear, trigonal planar, or tetrahedral, just to name a few. It's a rather elegant concept, don't you think?
Why Electron Pairs Matter
When we talk about "electron groups" or "electron pairs," we're talking about both the electrons that are shared in bonds (these are called bonding pairs) and those that are not shared (these are called lone pairs). Both types of electron groups take up space around the central atom, and both contribute to the overall electron geometry. However, lone pairs tend to push a bit harder than bonding pairs, which can slightly change the final molecular shape.
For instance, a lone pair on a central atom will occupy more space than a bonding pair, because it's only attracted to one nucleus, not two. This extra push can squeeze the bond angles between the atoms a little bit, making the molecule look slightly different from what you might expect if you only considered the bonding atoms. This distinction is really important when you're using your VSEPR chart to figure out the exact shape, as a matter of fact.
How the VSEPR Chart Helps
Starting with Lewis Structures
Before you can use a VSEPR chart, you really need a good Lewis structure for your molecule. This is your starting point, your blueprint. The Lewis structure shows all the atoms, how they're connected, and where all the valence electrons are, including those lone pairs. For example, when you draw the best Lewis structure for something like CH4 or H2O, you're setting yourself up to use the VSEPR chart correctly.
My text mentions "drawing the best Lewis structure for each of the following structures," and that's exactly right. Without an accurate Lewis structure, you won't know how many electron groups are around the central atom, and that information is absolutely vital for using the VSEPR chart. So, take your time with this first step; it really helps to get it right.
Counting Electron Domains
Once you have your Lewis structure, the next big step is to count the "electron domains" around the central atom. An electron domain is simply a region where electrons are found. This includes single bonds, double bonds, triple bonds, and lone pairs. Each of these counts as one electron domain, regardless of whether it's a single, double, or triple bond. So, a double bond, for example, is still just one electron domain.
This counting is super important because the number of electron domains directly tells you the electron geometry. If you have two electron domains, it's linear. Three, it's trigonal planar. Four, it's tetrahedral, and so on. This is where your VSEPR chart truly becomes a guide, showing you the electron arrangement based on this count, and it's a pretty neat system.
Distinguishing Electron and Molecular Geometry
Here's a key point that sometimes trips people up: there's a difference between electron geometry and molecular geometry. Electron geometry is about the arrangement of *all* electron domains (bonding and lone pairs) around the central atom. Molecular geometry, however, only describes the arrangement of the *atoms* themselves.
The VSEPR chart helps you determine both. First, you find the electron geometry based on the total number of electron domains. Then, you look at how many of those domains are bonding pairs and how many are lone pairs. The lone pairs affect the molecular geometry by taking up space but not having an atom attached. For instance, water (H2O) has four electron domains (two bonding pairs and two lone pairs), making its electron geometry tetrahedral, but its molecular geometry is bent. This distinction is very useful for accurately describing a molecule's true shape, you know.
Common Molecular Shapes from the VSEPR Chart
Linear and Trigonal Planar
Let's look at some common shapes you'll find on a VSEPR chart. When you have two electron domains around a central atom, they'll spread out to be as far apart as possible, which means they'll form a straight line. This shape is called linear, and the bond angle is 180 degrees. Carbon dioxide (CO2) is a good example of this, where the carbon is the central atom.
If there are three electron domains, they'll arrange themselves in a flat triangle. This is known as trigonal planar geometry. The bond angles here are typically 120 degrees. Boron trifluoride (BF3) is a classic example of a trigonal planar molecule. These shapes are quite straightforward to picture, and they're often the first ones you learn, so it's a good place to start, actually.
Tetrahedral and Its Variations
When you have four electron domains around a central atom, they'll arrange themselves in a tetrahedral shape. This is a 3D shape with bond angles of approximately 109.5 degrees. Methane (CH4) is the perfect example of a molecule with a tetrahedral molecular geometry, as it has four bonding pairs and no lone pairs.
But what if some of those four domains are lone pairs? This is where the VSEPR chart really shines. If you have three bonding pairs and one lone pair, the electron geometry is still tetrahedral, but the molecular geometry becomes trigonal pyramidal. Ammonia (NH3) fits this description. The lone pair pushes the bonding pairs closer together, making the angles a bit less than 109.5 degrees. And if you have two bonding pairs and two lone pairs, like in H2O, the molecular geometry becomes bent. These variations are really important to recognize, you know, as they show how lone pairs influence the final shape.
Trigonal Bipyramidal and Octahedral
For molecules with five electron domains, the electron geometry is trigonal bipyramidal. This shape has two distinct positions: axial and equatorial. Atoms in the equatorial plane are 120 degrees apart, while axial atoms are 90 degrees from the equatorial ones. Sulfur pentafluoride (SF5) is an example, though it's often more about phosphorus pentachloride (PCl5) for the pure trigonal bipyramidal form. Lone pairs can occupy equatorial positions to minimize repulsion, leading to shapes like seesaw, T-shaped, or linear.
With six electron domains, the electron geometry is octahedral. All positions are equivalent in an octahedral arrangement, and the bond angles are 90 degrees. Sulfur hexafluoride (SF6) is a good example of this symmetrical shape. If lone pairs are present, you can get square pyramidal or square planar molecular geometries. These higher-number electron domains can be a bit more complex, but the VSEPR chart still gives you a clear path to follow, which is quite helpful.
Putting the VSEPR Chart to Work: Examples
Methane (CH4): A Classic Example
Let's walk through an example from "My text," like CH4. First, you draw the Lewis structure for methane. You'll find that carbon is the central atom, and it's bonded to four hydrogen atoms. There are no lone pairs on the carbon atom. So, the carbon atom has four electron domains, all of which are bonding pairs.
Looking at your VSEPR chart, four electron domains mean the electron geometry is tetrahedral. Since all four domains are bonding pairs, the molecular geometry is also tetrahedral. This means the hydrogen atoms are arranged around the carbon atom at angles of about 109.5 degrees, giving it a very symmetrical, pyramid-like shape. It's a pretty straightforward application of the chart, actually.
Water (H2O): The Bent Shape
Next, let's consider H2O, also mentioned in "My text." When you draw the Lewis structure for water, oxygen is the central atom. It forms single bonds with two hydrogen atoms. Importantly, oxygen also has two lone pairs of electrons. So, around the central oxygen, you have a total of four electron domains: two bonding pairs and two lone pairs.
According to the VSEPR chart, four electron domains mean the electron geometry is tetrahedral. However, because two of those domains are lone pairs, they push the two hydrogen atoms closer together. This results in a "bent" molecular geometry, with a bond angle closer to 104.5 degrees, rather than the ideal 109.5 degrees of a perfect tetrahedron. This shows how those lone pairs truly influence the final shape, you know.
Ozone (O3): A Different Kind of Bent
Ozone (O3) is another interesting example from "My text." When you draw the Lewis structure for ozone, one oxygen atom is central. This central oxygen forms a double bond with one oxygen atom and a single bond with the other. It also has one lone pair. So, the central oxygen has three electron domains: two bonding domains (one double bond, one single bond) and one lone pair.
With three electron domains, the electron geometry is trigonal planar. But because one of those domains is a lone pair, the molecular geometry becomes bent. The bond angle in ozone is about 116.8 degrees, which is a bit less than the 120 degrees you'd expect for a perfect trigonal planar shape, again due to the lone pair's influence. It's another great illustration of how the VSEPR chart helps predict these specific arrangements, and it's quite a helpful tool.
Beyond Shape: Bond Angles and Polarity
Understanding Bond Angles
The VSEPR chart doesn't just tell you the general shape; it also gives you a good idea of the bond angles within the molecule. As "My text" suggests, you should "create a chart delineating the key information to determine the VSEPR model and be sure to include the predicted bond angles." These angles are determined by the repulsion between electron groups.
While the chart gives you ideal angles (like 109.5 for tetrahedral), remember that lone pairs can cause slight deviations. They exert a stronger repulsive force than bonding pairs, which can squeeze the angles between the atoms. So, while CH4 has perfect 109.5-degree angles, H2O's angles are a bit smaller. Knowing these predicted angles helps you draw more accurate 3D VSEPR diagrams, and it's quite an important detail, really.
Connecting to Molecular Polarity
The shape of a molecule, which you determine using the VSEPR chart, is absolutely key to understanding its polarity. "My text" asks, "How many of the following molecules are nonpolar?" and "How does the electronegativity of an element help to classify the polarity?" This is where VSEPR connects to a bigger picture.
A molecule's polarity depends on two things: the polarity of its individual bonds (which relates to electronegativity differences) and the overall molecular geometry. Even if a molecule has polar bonds, if its shape is perfectly symmetrical (like CH4, which is tetrahedral), the bond dipoles can cancel each other out, making the entire molecule nonpolar. However, if the shape is asymmetrical (like H2O, which is bent), the bond dipoles won't cancel, and the molecule will be polar. So, the VSEPR chart is really a vital step in figuring out if a molecule will be polar or nonpolar, and that's a pretty big deal for understanding how chemicals interact, you know.
Practical Tips for Using Your VSEPR Chart
Building Models and Using Online Tools
My text mentions, "Build each molecule using a model kit, or use molview.org (nb, This step is optional)." While it says optional, I'd really suggest doing it! Physically building a molecule with a model kit can make the 3D shapes click in your mind in a way that drawings sometimes can't. You can literally feel the angles and see how the atoms spread out.
If you don't have a model kit, online tools like MolView.org are fantastic. They let you spin molecules around, see them from different angles, and truly visualize the electron and molecular geometries. This hands-on or virtual experience makes using your VSEPR chart much more intuitive, and it's a very helpful way to learn, actually.
Creating Your Own Chart
The prompt also suggests, "Create a chart delineating the key information to determine the VSEPR model and be sure to include the predicted bond angles." This is an excellent idea! Making your own VSEPR chart helps you process and remember the information better than just looking at a pre-made one. Include columns for:
- Number of Electron Domains
- Number of Bonding Pairs
- Number of Lone Pairs
- Electron Geometry
- Molecular Geometry
- Predicted Bond Angles
- Examples (like CH4, H2O, O3)
This personal chart will become your go-to reference, and it really helps solidify your understanding. It's a great way to study, too, if you think about it.
Frequently Asked Questions About the VSEPR Chart
What is the VSEPR theory?
The VSEPR theory is a way to predict the three-dimensional shape of molecules. It works on the simple idea that electron groups around a central atom will push each other away to get as much space as possible. This repulsion determines the molecule's overall shape, and it's a very useful concept in chemistry.
How do you use a VSEPR chart to determine molecular geometry?
To use a VSEPR chart, first, you need to draw the molecule's Lewis structure. Then, count the total number of electron domains (bonding pairs and lone pairs) around the central atom. This count tells you the electron geometry. Next, look at how many of those domains are actual bonds and how many are lone pairs. The VSEPR chart then guides you to the specific molecular geometry based on these numbers, which is pretty straightforward.
What is the difference between electron geometry and molecular geometry?
Electron geometry describes the arrangement of all electron groups (both bonding and lone pairs) around the central atom. Molecular geometry, on the other hand, describes only the arrangement of the atoms themselves. Lone pairs take up space and influence the electron geometry, but they are not "seen" as part of the molecular shape. For example, H2O has a tetrahedral electron geometry but a bent molecular geometry because of its two lone pairs, and it's an important distinction to grasp.
A Final Thought on the VSEPR Chart
The VSEPR chart is a powerful tool for anyone studying chemistry. It bridges the gap between a flat chemical formula and the real, three-dimensional world of molecules. By using it, you can predict shapes, understand bond angles, and even figure out if a molecule will be polar. This knowledge helps explain so much about how substances behave, and it's a skill that will really help you in your chemistry journey. So, keep practicing those Lewis structures, keep counting those electron domains, and let your VSEPR chart guide you to a better grasp of molecular shapes. You can learn more about molecular shapes on our site, and for a deeper look at chemical bonding, that page has some great insights too.

Vsepr Chart

VSEPR Chart | Molecular Geometry Worksheet Answer Key
Vsepr Model